Drug Development Process
Every new drug treatment must follow a regulatory approval process. Once a drug is determined to provide benefits, it must be determined whether the benefits outweigh any known or potential risks to the patient. Before using new drugs on humans, researchers investigate the effects of the drug and test drug delivery and dosing in vivo before any humans are treated. Based on the results, an investigational new drug application is made to the appropriate regulatory authority. Once approved, the new drug may be given to patients.
Gene replacement therapy treatments go through several years of rigorous pre-clinical testing in order to establish the efficacy and safety of the chosen formulation of the gene therapy medicine. Rare Trait US funded all of the necessary pre-clinical work related to the AGU gene therapy and together, we are ready to move to the next stage – a clinical trial for our children. Our progress so far is indicated by the orange arrow on the following chart:
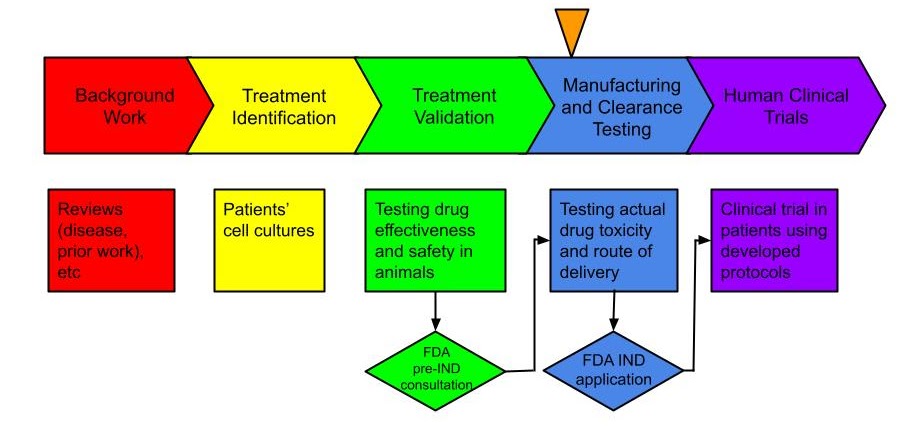
Here’s what’s coming up in the near future. This is how your donations are being spent:
- Manufacture gene therapy drug (AGA gene + delivery vector) (DONE!)
- Conduct toxicology and dose/biodistribution study (underway)
- Conduct drug clearance testing (DONE!)
- Submit results of all of the above to FDA for IND (Investigational New Drug) Application
- Start clinical trials in AGU patients
You can see how far we’ve come and how close we are to the last piece in the puzzle of curing AGU!
